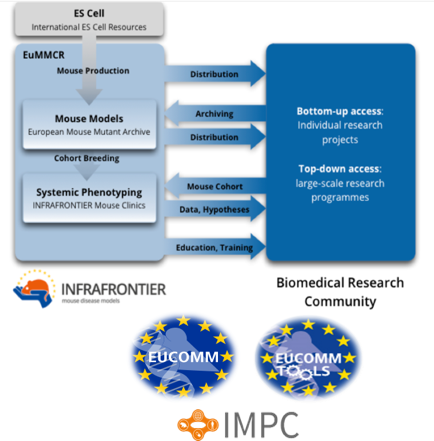
Overview
The Monterotondo Mouse Clinic participates in the INFRAFRONTIER-EMMA European Network Infrastructure (Landmark Project of the European Strategy Forum on Research Infrastructures – ESFRI – Roadmap) which aims at building a world-class research infrastructure that provides the international biomedical research community with the tools needed to unravel the role of gene function in human diseases.
The Infrafrontier-EMMA International Networks and Consortia are coordinated and managed by Infrafrontier Gmbh-Helmholtz Zentrum Muenchen and comprise the leading biomedical research Institutions of most European countries, Israel and Canada, including CNR as the Italian promotor and founding member.
https://www.infrafrontier.eu/about-us/infrafrontier-partners/
INFRAFRONTIER-EMMA Mission
- Shaping the European Research Area in the field of mouse functional genomics and thereby make an important contribution to the study of human disease.
- Setting reference standards for systemic phenotyping of mouse models and for archiving and distribution of mouse mutants in Europe.
- Offering highest-quality services and cutting-edge technologies provided by the leading labs in Europe.
- Disseminating knowledge by state-of-the-art training courses.
Objectives
- Providing access to mouse models, data, and scientific platforms and services to study the functional role of the genome in human health and disease.
- Archiving and distribution of scientifically valuable mouse strains through the European Mouse Mutant Archive (EMMA), one of the world’s leading mouse repositories.
- Providing access to a whole-organism, systemic analysis of genotype-phenotype interactions using cutting-edge analytical and diagnostic methodology in the INFRAFRONTIER mouse clinics.
- Providing bottom-up access for individual scientists and research groups and top-down capacities for large-scale international initiatives such as the International Mouse Phenotyping Consortium (IMPC).
Animal welfare and ethics
The Health and Welfare of animals are of paramount importance to INFRAFRONTIER-EMMA, and this focus has helped to shape the policies and the design of the mouse facilities of network partners.
Health monitoring procedures
Animals generated and distributed by EMMA are bred in SPF (Specific Pathogen Free) barriered facilities in which all materials are sterilized before entry.
CNR-IBBC Monterotondo’s mutant strain production unit (MMP) since 2008 has participated in the EUCOMM (European Conditional Mouse Mutagenesis) project, the cornerstone of the International Knockout Mouse Consortium (IKMC). So far, the unit has produced more than 200 mutant mouse lines, by ES cells recombination and blastocyst injection and CRISPR/Cas technologies, providing them for the large-scale mouse phenotyping projects EUMODIC (European Mouse Disease Clinic) and IMPC (International Mouse Phenotype Consortium). The unit has also participated in the EUCOMM-Tools project and has produced over 50 cre recombinase-driver mouse mutant lines , as requested by the relevant work-package. Most of these lines are already cryopreserved and distributed by INFRAFRONTIER-EMMA, with MMP participating in their integrated core transgenic facilities.
Health reporting
Before receiving any mice from EMMA you will be sent a recent (less than three months old) health report prepared in accordance with the FELASA recommendations. This health report will give details of the agents tested, the number of animals tested and the analytical methods used.
Sample health reports from each of EMMA’s archiving / distribution centres can be viewed on the INFRAFRONTIER website. If you require any further information please contact the archiving / distribution centre handling your request
The European Mutant Mouse Archive (EMMA) currently comprises over 8400 mutant mouse lines that are cryopreserved as frozen sperm and/or embryos. These mutants carry targeted (ES cells recombination- or endonuclease-mediated), transgenic, chemically or radiation-induced, spontaneous and other types of mutations. A major part of the repository consists of strains deposited into EMMA by individual researchers.
In addition, EMMA’s partners have been and are actively participating in the production of major collections of mutant models include, for instance:
- Lines generated by the International Knockout Mouse Consortium (IKMC) and International Mouse Phenotyping Consortium (IMPC) large-scale, standardized production and phenotyping projects. These strains are available from EMMA. For more information about these lines, please have a look at the following documents: allele overview, allele conversion guide and nomenclature for mutant alleles generated by IKMC.
- GEMM collection (MRC Genome Editing Mice for Medicine Programme)
- PHENOMIN-ICS Nuclear Receptors mutant collection.
- The Wellcome Trust Knockout Mouse Resource with strains generated by Deltagen and Lexicon
- INFRAFRONTIER-EMMA collection of strains carrying mutations of genes involved in more than 1600 rare diseases
- INFRAFRONTIER-EMMA collection of strains carrying mutations of genes whose human orthologues are involved in COVID19/ SARS-CoV-2 infection
- INFRAFRONTIER-EMMA collection of strains carrying mutations of genes involved in many different types of cancer
Research tool collections include:
- More than 390 cre recombinase driver lines, with over 200 Cre drivers produced by the EUCOMMTools project
- FLP deleter lines
- Lines using the TET expression systems
Specific disease models
Additionally, EMMA has manually curated the nomenclature, description etc. of several archived mouse strains that have been reported to constitute specific disease models. The users can now look for diseases of interest and check if appropriate mouse models, suitable for their research, are available.
https://www.infrafrontier.eu/services/systemic-phenotyping
The INFRAFRONTIER partners offer the production and phenotypic examination of specific mouse mutant models using a broad and standardised phenotypic check-up covering key research areas such as behaviour, clinical chemistry, immunology, energy metabolism, and lung function amongst others. If phenotypes are uncovered by the primary assays, mouse mutants can be subjected to more detailed analyses in hypothesis driven and disease focussed phenotyping pipelines.
This specialised phenotyping services can provide access to a comprehensive panel of phenotyping tests, relying on standardized and customized protocols in key therapeutic areas.

Depositing mice into the EMMA repository
www.infrafrontier.eu/emma/cryopreservation
www.infrafrontier.eu/submissionForm/#!submissionTermsView
Online submission
Prospective depositors will use the online submission form to submit their strains to EMMA. The submission form captures information on depositor and owner, mutation(s), phenotype, genetic background, literature references, strain characterization, scientific interest, intellectual property rights and some additional information that is needed for handling the mice. Receipt of the online submission form will be confirmed by an automatic e-mail notification.
Evaluation
Mouse strains submitted for archiving are evaluated by EMMA’s external Evaluation Committee. The committee is composed of experts in the field of mouse genetics. They evaluate each submission to the EMMA repository, thereby ensuring that the research community has access to valuable mouse mutant strains with significance for current and future genetic research. If the information provided through the online submission form is clear enough, EMMA will inform depositors about the outcome of the evaluation within 60 days.
The EMMA cryopreservation service is free of charge. However, depositors have to cover the actual costs for shipping material to the EMMA facilities.
Strain ordering from the EMMA Repository
www.infrafrontier.eu/emma/strain-distribution
www.infrafrontier.eu/search
EMMA strains are cryopreserved as frozen embryos and/or sperm. Highly demanded EMMA strains are maintained on shelf to facilitate fast delivery to the users. For all other EMMA strains a rederivation service can be provided.
INFRAFRONTIER-EMMA training and consulting services
www.infrafrontier.eu/training-events
The INFRAFRONTIER-EMMA Research Infrastructure and its partners offer a wide range of state-of-the-art training opportunities. Mouse clinics in different European countries provide outstanding trainings in first-line mouse phenotyping as well as specialised phenotyping courses covering e.g. mouse osteology and mouse blood and lymphatic vessel phenotyping, as well as many other research areas. The INFRAFRONTIER Courses also cover unique training opportunities in mouse model development as well as hands on cryopreservation training workshops, offered by EMMA partners since many years.
INFRAFRONTIER-EMMA Resources and Services: Details and FAQs
www.infrafrontier.eu/services
www.infrafrontier.eu/emma/strain-distribution/#FAQs
www.infrafrontier.eu/emma/cryopreservation/#FAQs
How to order
Please visit the EMMA strain list, find your strain of interest, open the strain details by using the ‘+’ toggle, then click the ‘Order’ button. EMMA will contact you within two weeks after receipt of your EMMA Mutant Request Form. For more information about the order process please check the FAQs section.
Members and contacts
|
mouse.resources@emma.cnr.it Mouse Production: Data Curation |
Mutant Model phenotyping
|
Publications
https://www.infrafrontier.eu/knowledgebase/bibliography
Phenotypic analysis pipeline of murine lines for the analysis of physiological/functional parameters of control and mutant male and female mice following IMPC guidelines (https://www.mousephenotype.org):
1. Experimental design, assignment to phenotyping groups, mouse colony management (breeding scheme, genotyping strategy, animal maintenance).
2. Developmental/embryo phenotyping analysis.
Growth morphology by light microscopy and X-ray imaging and histological analysis.
3. Phenotyping analysis of adult mutant animals:
Behavioural phenotyping.
Metabolic analysis: Glucose Tolerance Test, blood chemistry.
Immunophenotyping: Contact hypersensitivity test and FACS analysis.
4. Analysis of organs and tissues ex vivo (Light and X-ray Imaging). Histopathology
5. Whole mouse X-ray imaging in vivo.
Members:
Behavioural phenotyping: Mandillo S; Golini E.
Metabolic analysis and Contact Hypersensitivity: Ermakova O
FACS analysis: Putti S
Light imaging; histology, pathology: La Sala G; Di Pietro C; Marazziti D
X-ray imaging, Orsini T.

