Group Leader: Maurizia Caruso
Topics: Muscle Biology and Pathology
Keywords: Muscle metabolism, Cell cycle regulators, muscular dystrophy
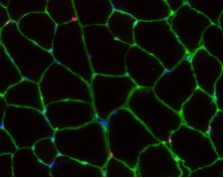
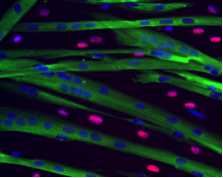
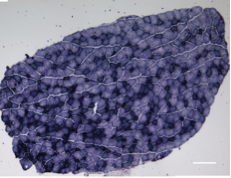
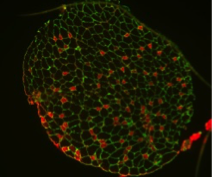
A long-standing research interest in our lab has been focused on the crosstalk between cell cycle regulation and the differentiation program of skeletal muscle stem cells, with specific attention to the role of D-type cyclins in these processes. In past years our studies have revealed a unique function played by cyclin D3 in the control of the developmental program of skeletal muscle precursor cells both in vitro and in vivo. Recently, we have uncovered a novel cell cycle-independent role for cyclin D3 in regulating the contractile and metabolic properties of skeletal muscle fibers and whole-body energy metabolism. Furthermore, we have demonstrated that genetic inactivation of cyclin D3 in the mdx mouse model of Duchenne Muscular Dystrophy (DMD) ameliorates the disease phenotype, by promoting a slower, more oxidative profile of muscle fibers that is more resistant to dystrophic pathology.
- In view of a possible translational approach, we are now evaluating the therapeutic efficacy of targeting cyclin D3 activity in adult mdx mice when the signs of disease are already evident.
A related research interest is centered on the regulatory crosstalk between skeletal muscle and brain. Skeletal muscle is the most abundant tissue of the body and a major metabolic organ. Furthermore, this tissue produces and secretes growth factors and cytokines (commonly termed myokines) that modulate systemic physiology. Skeletal muscle may crosstalk with brain via direct muscle-nerve interaction and release of metabolites and myokines in response to muscle contraction, diet-derived nutrients, and stress. There is evidence that myokines and other factors secreted by skeletal muscle contribute to the regulation of neurogenesis, memory and learning and that chronic muscle disorders can compromise hippocampal function.
- Our ongoing studies aim to evaluate the potential positive impact of external stimuli that influence pathways involved in energy metabolism (natural bioactive dietary compounds, exercise etc) on muscle dysfunctions and hippocampal neurogenesis defects in mouse models of muscular dystrophy and aging.
IBBC Collaborators: Laura Micheli, Felice Tirone, Siro Luvisetto
Key pubblications
Micheli L, Bertini L, Bonato A, Villanova N, Caruso C, Caruso M, Bernini R, Tirone F. (2023) Role of Hydroxytyrosol and Oleuropein in the Prevention of Aging and Related Disorders: Focus on Neurodegeneration, Skeletal Muscle Dysfunction and Gut Microbiota. Nutrients. 15:1767. doi: 10.3390/nu15071767.
Bonato A, Raparelli G, Luvisetto S, Forconi F, Cosentino M, Tirone F, Rizzuto E, Caruso M. (2023) Cyclin D3 deficiency promotes a slower, more oxidative skeletal muscle phenotype and ameliorates pathophysiology in the mdx mouse model of Duchenne muscular dystrophy. FASEB J. 37:e23025. doi: 10.1096/fj.202201769R.
Belli R, Bonato A, De Angelis L, Mirabilii S, Ricciardi MR, Tafuri A, Molfino A, Leigheb M, Costelli P, Caruso M, Muscaritoli M and Ferraro E. (2019) Metabolic Reprogramming Promotes Myogenesis During Aging. Frontiers in Physiology. 10:897. doi: 10.3389/fphys.2019.00897.
Giannattasio S, Giacovazzo G, Bonato A, Caruso C, Luvisetto S, Coccurello R and Caruso M. (2018) Lack of cyclin D3 induces skeletal muscle fiber-type shifting, increased endurance performance and hypermetabolism. Scientific Reports. 8:12792.doi: 10.1038/s41598-018-31090-5
Albini, S., Coutinho Toto, P., Dall’Agnese, A., Malecova, B., Cenciarelli, C., Felsani, A., Caruso, M., Bultman, S.J., Puri, PL. (2015) Brahma is required for cell cycle arrest and late muscle gene expression during skeletal myogenesis. EMBO Rep., 16:1037-50. doi: 10.15252/embr.201540159).
De Luca G, Ferretti R, Bruschi M, Mezzaroma E and Caruso M. (2013) Cyclin D3 critically regulates the balance between self-renewal and differentiation in skeletal muscle stem cells. Stem Cells 31: 2478-2491.
Orcid: http://orcid.org/0000-0001-5445-6343
Research Group
Post-doctoral fellow:
Agnese Bonato
bonato.agnese@gmail.com
Research fellow
Giada Raparelli
giadaraparelli@icloud.com
Programs & resourcers
2024-2028: Associazione per le Neuroscienze Giuseppe Moruzzi. “Attivazione mediante stimoli neurogenici delle cellule staminali neurali nelle nicchie neurogeniche adulte e nel cervelletto: studio della fisiopatologia correlata e del ruolo dell’asse cervello-muscolo”, Role: Partner
2023-2025: (CNR-FOE 2021)/ Progetto Nutrage Attività IBBC-5 “Effetto dei polifenoli dell’olio di oliva sulle cellule staminali delle nicchie neurogeniche e muscolari e sul microbiota intestinale in modelli murini di invecchiamento”, Role: Partner
2020-2023: Duchenne Parent Project, Netherlands project N. 19018 “Evaluation of cyclin D3 as a potential target to remodel dystrophic muscle toward the slow, oxidative phenotype”, Role: Principal investigator
2020-2024: Lazio Innova POR FESR LAZIO Programmazione 2014 – 2020, Project N. A0375-2020-36407 coordinated project “Declino cognitivo, perdita di funzionalità muscolare e alterazioni del microbiota intestinale associati all¹invecchiamento: effetti protettivi dell¹idrossitirosolo, componente dell¹olio di oliva e di estratti da sottoprodotti oleari”, Role: Partner
2020-2025: Fondazione Adriano Buzzati-Traverso “Studio dell’attivazione da parte di stimoli neurogenici delle cellule staminali neurali nelle nicchie neurogeniche adulte e nel cervelletto, e della fisiopatologia inerente a questi processi”, Role: Participant
2009-2013: Telethon project N. GGP08126: “Role of cyclin D3 in satellite cell function and muscle regeneration”, Role: Principal investigator
