
Elvira De Leonibus
Research Areas: Neuroscience
THE NEUROBIOLOGY OF MEMORY CAPACITY
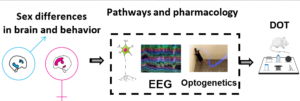 MEMORY CAPACITY is the number of items that a subject can remember for a short-time interval,. We have developed novel behavioural tasks (the DOT/IOT in Sannino et al L&M2012) to address how the brain processes different amount of information in normal and pathological conditions, when and how a direct transfer into long-term memory occurs and whether these processes are developmental regulated and sex-related
MEMORY CAPACITY is the number of items that a subject can remember for a short-time interval,. We have developed novel behavioural tasks (the DOT/IOT in Sannino et al L&M2012) to address how the brain processes different amount of information in normal and pathological conditions, when and how a direct transfer into long-term memory occurs and whether these processes are developmental regulated and sex-related
TARGETING EARLY DISEASE MECHANISMS IN AGING AND NEURODEGENERATIVE DISORDERS
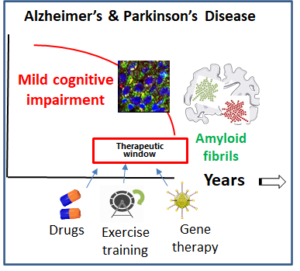
Neurodegenerative disorders are characterized by progressive neuropathological processes that last decades before that a conspicuous loss of neurons can be detected. Therefore, there is a large therapeutic window to find early subtle behavioural and cognitive symptoms that allow early diagnosis and the discovery of the disease mechanisms leading to neuronal loss.
We use a top-down approach: we move from the early occurring behavioural defects to the neuronal, biochemical and molecular mechanisms responsible for it, to finally target them with small molecules (authophagy enhancers, dopaminergic drugs, etc) and provide proof-of concept pre-clinical evidence of their therapeutic efficacy. Using this top-down research strategy, we are working on Ageing (which is the first known cause of cell damage), Alzheimer’ disease and Parkinson’ disease.
TARGETING EARLY DISEASE MECHANISMS LYSOSOMAL STORAGE DISORDERS
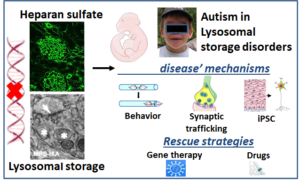 In lysosomal storage disorders, which are a group of inherited diseases due to defective degradation of glycosaminoglycans, the “sugars of the brain”, neurodegeneration runs in parallel with neurodevelopmental disorders, such as autism. We are studying the developmental trajectory and disease mechanisms leading to early occurring autistic symptoms and the shift to neurodegeneration in SanFilippo syndrome.
In lysosomal storage disorders, which are a group of inherited diseases due to defective degradation of glycosaminoglycans, the “sugars of the brain”, neurodegeneration runs in parallel with neurodevelopmental disorders, such as autism. We are studying the developmental trajectory and disease mechanisms leading to early occurring autistic symptoms and the shift to neurodegeneration in SanFilippo syndrome.
EDUCATION:
1999 Master Degree in Psychology, University of Rome “La Sapienza”, Rome, Italy (final mark: 110/110 cum laude)
1999-2002 PhD, School of Psychobiology and Psychopharmacology, University of Rome “La Sapienza”, Rome, Italy
CURRENT POSITIONS
2018 – Group Leader (I Ricercatore), Neuropsychopharmacology Laboratory, Institute of Cellular Biology and Biochemistry (IBBC) (previously called Institute of Cellular Biology and Neurobiology), Monterotondo (RM), Italy
2011 – Group Leader (Faculty-Contract), Behavioral Genetics laboratory, Telethon Institute of Genetics and Medicine (TIGEM), Naples, Italy
2007 – Head, Animal Behavioral Facility, Telethon Institute of Genetics and Medicine (TIGEM), Pozzuoli (Naples), Italy
PREVIOUS POSITIONS
July 2018 Visiting scientist, Biomedical Center, Medical Charles University of Pilsen, Chez Republic. EMBO fellowship
2010-2018 Group Leader, Neuropsychopharmacology Laboratory, Institute of Genetics and Biophysics (IGB-CNR) “Adriano Buzzati-Traverso”, Naples, Italy
2010-2018 Head of the joint Telethon Institute of Genetics and Medicine (TIGEM)- Institute of Genetics and Biophysics (IGB-CNR) behavioral facility, Naples, Italy
2006-2007 Post Doctoral fellow, Psychobiology Laboratory, Dept. Institute of Genetics and Biophysics (IGB-CNR)Darwin”, Mathematics and Natural Sciences Department, University of Rome “La Sapienza”, Rome, Italy
2004-2006 Clinical Training, Skinner Institute, Rome, Italy
2005 Visiting scientist (one month), Neurobiologie de la Cognition Lab, CNRS, Marseille, France
2003-2004 Associate Researcher (Rotary Club Research Grant), Neuropsychopharmacology Laboratory, Catholic University of Nijmegen, Nijmegen, Netherlands
Honours/Awards:
2018 EMBO short-term Fellowship as visiting Scientist at the Biomedical Center, Medical Charles University of Pilsen, Chez Republic.
2010 Young investigator grant award, American Alzheimer’ Association
2008 Annual prize for scientific achievements in psychobiology from the magazine “Mente e Cervello” (part of the Italian version of the American magazine “Scientific American”)
2007 Research Fellow from the Telethon Foundation
2007 IBRO travel-grant to attend the 37th Annual Meeting of Society of Neuroscience, San Diego (California), USA, November 2005
2005 IBRO travel-grant to attend the 35th Annual Meeting of Society of Neuroscience, Washington DC, USA, November 2005
2004 IBRO travel-grant to attend the 9th Multidisciplinary International Conference of Biological Psychiatry “Stress and Behavior”, San Petersburg (Russia), May 2005
2003 Award as a one-year scholar Ambassador of Rotary International, Rotary International, Evanston (USA), at the Catholic University of Nijmegen, Nijmegen, the Netherlands (Dr. A.B. Ellenbroek and A.L. Cools).
2002 Fellowship for training in foreign country, University of Rome “la Sapienza”, Rome Italy
1999-2002 PhD fellowship, University of Rome “La Sapienza”, Rome, Italy “PhD in Psychobiology and Psychopharmacology”
1996 Undergraduate fellowship at the Faculty of Literature, University of Rome “La Sapienza”, Rome, Italy.
- De Risi M, Tufano M, Alvino FG, Ferraro MG, Torromino G, Gigante Y, Monfregola J, Marrocco E, Pulcrano S, Tunisi L, Lubrano C, Papy-Garcia D, Tuchman Y, Salleo A, Santoro F, Bellenchi GC, Cristino L, Ballabio A, Fraldi A, De Leonibus E. Altered heparan sulfate metabolism during development triggers dopamine-dependent autistic-behaviours in models of lysosomal storage disorders. Nat Commun. 2021 Jun 9;12(1):3495. doi: 10.1038/s41467-021-23903- Manuscript featured by Editors highlights: https://www.nature.com/collections/mjkksldswr
- Torromino G., Maggi A., De Leonibus E. Estrogens-dependent hippocampal wiring as a risk factor for ageing-related dementia in women. Feb;197:101895, 2021 Progress in Neurobiology.
- De Risi M, Torromino G, Tufano M, Moriceau S, Rivogorda M, Pignataro A, Ammassari_Teule M, Gardoni F, Middei S, Mele A, Oury F, De Leonibus E. Mechanisms through which autophagy regulates memory capacity in ageing”. Sep;19(9):e13189, 2020, Aging Cell.
- Giordano NC*, Iemolo A*, Mancini M, Cacace F, De Risi M, Latagliata EC, Ghiglieri V, Bellenchi GC, Puglisi-Allegra S, Calabresi P, Picconi B, De Leonibus E. Dopamine active transporter mediates learning-induced shift in striatal plasticity: relevance for Parkinson’s disease. Feb 1;141(2):505-520, 2018, Brain.
- Flore G, Di Ruberto G, Sannino S, Russo F, Illingworth E, Studer M, De Leonibus E. Gradient COUP-TFI Expression is Required for Proper Functional Organization of the Hippocampal Septo-Temporal Longitudinal Axis. Feb 1;27(2):1629-1643, 2017, Cerebral Cortex .
Invited book chapter
Iemolo A, De Risi M, De Leonibus E. 2015. Role of Dopamine in memory consolidation. In: Sakakibara, M and Ito E eds. Memory Consolidation. Nova Science Publisher.
*Identical contribution to the study
Peer-reviewed manuscripts (underlined Last, first or corresponding author)
- Indrieri A, Pizzarelli R, Franco B, De Leonibus E. Synaptic and mitocondrial dysfunctions in Parkinsonian eyes, Topic: Seeing Beyond the Eye: The Brain Connection. Oct 19;14:567129, 2020, Frontiers in Neuroscience.
- Torromino G., Maggi A., De Leonibus E. Estrogens-dependent hippocampal wiring as a risk factor for ageing-related dementia in women. Feb;197:101895, 2021 Progress in Neurobiology.
- De Risi M, Torromino G, Tufano M, Moriceau S, Rivogorda M, Pignataro A, Ammassari_Teule M, Gardoni F, Middei S, Mele A, Oury F, De Leonibus E. Mechanisms through which autophagy regulates memory capacity in ageing”. Sep;19(9):e13189, 2020, Aging Cell.
- Rinaldi A*, De Leonibus E*, Cifra A, Torromino G, Minicocci E, De Sanctis E, Lopez‑Pedrajas R, Oliverio A and Mele A. Flexible use of allocentric and egocentric spatial memories activates differential neural networks in mice. Jul 9;10(1):11338, 2020, Scientific Reports. *Identical Contribution
- Marrocco E, Indrieri A, Esposito F, Tarallo V, Carboncino A, Alvino FG, De Risi M, , Franco B, Auricchio A, De Leonibus E. Alpha-synuclein in the retina leads to degeneration of dopamine amacrine cells impairing vision. 10(1):9619, 2020, Scientific Reports
- Sorrentino NC, Cacace V*, De Risi* M, Maffia V*, Strollo S, Tedesco N, Nusco E, Roagnoli N, Bacci ML, Huang Y, Liu N, Kalled SL, Choi VW, De Leonibus# E, Fraldi# Improved secretion and post-translational activation of sulfamidase enhances the bio-distribution and the therapeutic potential of sulfamidase for the treatment of MPS-IIIA. Oct 28;15:333-342, 2019, Molecular Therapy – Methods & Clinical Development.#Co-corresponding author
- Monaco A, Maffia V, Sorrentino NC, Sambri I, Ezhova Y, Giuliano T, Cace V, Nusco E, De Risi M, De Leonibus E, Schrader T, Klanrner FG, Bitan G, Fraldi A. Inhibiting amyloid protein aggergation relieves lysosomal-autophagic dysfunction and protects against neurodegeneration in a severe lysosomal storage disease 28(4):1167-1176, 2020, Embo Molecular Medicine.
- Indrieri A, Carrella S, Romano A, Spaziano A, Marrocco E, Fernandez-Vizarra, Barbato S, Pizzo M, Ezhova Y, Golia FM, Ciampi L, Tammaro R, Henao-Mejia J, Williams A , Flavell RA, De Leonibus E, Zeviani M, Surace EM, Banfi S, Franco B. miR-181a/b Downregulation ameliorates Mitochondrial Diseases by coordinated activation of mitochondrial biogenesis and mitophagy. May;11(5), 2019, Embo Molecular Medicine.
- Durante V, de Iure A, Loffredo V, Vaikath N, De Risi M,Paciotti S, Quiroga-Varela A, Chiasserini D, Mellone M, Mazzocchetti P, Calabrese V, Campanelli F, Mechelli A, Di Filippo M, Ghiglieri V, Picconi B, El-Agnaf OM, De Leonibus E, Gardoni F, Tozzi A, Calabresi P.. Alpha-synuclein targets GluN2A NMDA receptor subunit causing striatal synaptic dysfunction and visuo-spatial memory alteration. May 1;142(5):1365-1385, 2019, Brain.
- Olivito L*, De Risi M*, Russo F, Cecere A, De Leonibus E. Role of dopamine receptors in working memory capacity. Nov 2;359:197-205, 2018, Behav Brain Res.
- Iannotti FA, Pagano E, Moriello AS, Alvino FG, Sorrentino NC, D’Orsi L, Gazzerro E, Capasso R, De Leonibus E, De Petrocellis L, Di Marzo V. Effects of non-euphoric plant cannabinoids on muscle quality and performance of dystrophic mdx mice. Aug 3., 2018, Br J Pharmacol. [Epub ahead of print].
- Miniaci MC, De Leonibus E. Missing the egocentric spatial frame: a blank on the map. Feb 9;7:168, 2018, F1000Res (Invited Review).
- Picconi B, De Leonibus E, Calabresi P. Synaptic plasticity and levodopa induced dyskinesia: electrophysiological and structural abnormalities. Special issue on Parkinson disease, Feb 28. 2018, Journal Neural Transmission (Invited Review).
- Giordano NC*, Iemolo A*, Mancini M, Cacace F, De Risi M, Latagliata EC, Ghiglieri V, Bellenchi GC, Puglisi-Allegra S, Calabresi P, Picconi B, De Leonibus E. Dopamine active transporter mediates learning-induced shift in striatal plasticity: relevance for Parkinson’s disease. Feb 1;141(2):505-520, 2018,
- Gatto F, Rossi B, Tarallo T, Polishchuk E, Polishchuk R, Carrella A, Nusco E, Alvino FG, Iacobellis F, De Leonibus E, Auricchio A, Diez-Roux G, Ballabio A, Parenti G. AAV-mediated TFEB gene delivery ameliorates muscle pathology and function in the murine model of Pompe Disease. 8;7(1):15089, 2017, Scientific Reports,
- Viggiano D, Speranza L, Crispino M, Bellenchi GC, Di Porzio U, Iemolo A, De Leonibus E, Volpicelli F, Perrone-Capano C. Information content of dendritic spines after motor learning. Jan 15;336:256-260, 2017, Behavior Brain Research.
- Sambri I, D’Alessio R, Ezhova Y, Sorrentino NC, Nusco E, De Risi M, Cataldi M, Annunziato L, De Leonibus E, Fraldi A. Re-establishing presynaptic function via cysteine string protein-α prevents neurodegeneration in lysosomal storage disorders. Jan;9(1):112-132, 2017, EMBO Mol Med. Flore G, Di Ruberto G, Sannino S, Russo F, Illingworth E, Studer M, De Leonibus E. Gradient COUP-TFI Expression is Required for Proper Functional Organization of the Hippocampal Septo-Temporal Longitudinal Axis. Feb 1;27(2):1629-1643, 2017, Cerebral Cortex.
- Olivito L, Saccone P, Perri V, Bachman J, Fragapane P, Mele A, Huganir RL, De Leonibus E. Phosphorylation of the AMPA receptor GluA1 subunit regulates memory load capacity. Nov 8. 2014 [Epub ahead of print] Brain Structure Function.
- Ferla R, Claudiani P, Cotugno G, Saccone P, De Leonibus E, Auricchio A. Gene therapy overcomes the need for multiple infusions of enzyme replacement therapy in a mouse model of lysosomal storage disease. Jul;25(7):609-18, 2014, Human Gene Therapy.
- Saccone P, Cotugno G, Russo F, Mastrogiacomo R, Tessitore A, Auricchio A, De Leonibus E. Behavioural characterization of an animal model of Maroteaux-Lamy syndrome (or Mucopolysaccharidosis VI). Jan 10;4:3644, 2014, Scientific Reports.
- Sorrentino NC, D’Orsi L, Nusco E, Sambri I, Spampanato C, Polishchuk E, Saccone P, De Leonibus E, Ballabio A, Fraldi A. A highly secreted sulfamidase engineered to cross the blood-brain barrier corrects the CNS pathology of mice with mucopolysaccharidoses type IIIA. 2013, May;5(5):675-90, EMBO Molecular Medicine
- Managò F, Lopez S, Oliverio A, Amalric M, Mele A, De Leonibus E. Interaction between the mGlu receptors 5 antagonist, MPEP, and amphetamine on memory and motor functions in mice. Nov Epub ahead of print, 2012, Psychopharmacology.
- Murdocca M, Malgieri A, Luchetti A, Saieva L, Dobrowolny G, De Leonibus E, Filareto A, Quitadamo MC, Novelli G, Musarò A, Sangiuolo F. IPLEX administration improves motor neuron survival and ameliorates motor functions in a severe mouse model of spinal muscular atrophy. Sep 25;18:1076-85, 2012, Mol Med.
- Sannino S*, Russo F*, Torromino G, Pendolino V, Calabresi P, De Leonibus E. Role of the hippocampus in object memory load. 19: 211-18, 2012, Learning & Memory.
- Rotundo IL, Faraso S, De Leonibus E, Nigro G, Vitiello C, Lancioni A, Di Napoli D., Castaldo S, Russo V, Russo F, Piluso S, Auricchio A, Nigro V. Worsening of cardiomyopathy using Deflazacort in an animal model rescued by gene therapy. 6: e24729, 2011, Plos One.
- Lodato S*, Tomassy GS*, De Leonibus E*, Yoryani GU, Andolfi G, Armentano M., Gaztelu JM, Arlotta P, Mendez de la Prida L, Studer M. Loss of COUP-TF1 function alters the balance of CGE- and MGE-derived interneurons resulting in an epilepsy resistant phenotype. 31(12):4650-62, 2011, Journal Neuroscience.
- De Leonibus E*, Costantini VJA*, Massaro A, Vanni V, Mandolesi G, Luvisetto S, Pavone F, Oliverio A, Mele A. Cognitive and neural determinants of response strategy in the dual-solution plusmaze task. 18(4):241-4, 2011, Learning & Memory.
- Spampanato C*, De Leonibus E*, Dama P, Gargiulo A, Fraldi A, Sorrentino CN, Russo F, Nusco E, Auricchio A, Surace EM, Ballabio Efficacy of a combined intracerebral and systemic gene delivery approach for the treatment of a severe lysosomal storage disorder. 19(5):860-9, 2011, Human Molecular Genetics.
- Cotugno G, Annunziata A, Tessitore A, O’Malley T, Capalbo A, Faella A, Bartolomeo R, O’Donnell P, Wang P, Russo F, Sleeper MM, Knox VW, Fernandez S, Levanduski L, Hopwood J, De Leonibus E, Haskins M, Auricchio A. Long-term amelioration of feline MPS VI after AAV-mediated liver gene transfer. 19(3):461-9, 2011, Therapy.
- Lancioni A, Pizzo M, Fontanella B, Ferrentino R, De Leonibus E, Meroni G. Lack of Mid1, the mouse ortholog of the Opitz Syndrome gene, causes abnormal development of the anterior cerebellar vermis. 30(8):2880-7, 2010, Journal Neuroscience.
- Tomassy GS*, De Leonibus E*, Jabaudon D*, Lodato S, Alfano C. Mele A, Macklis JD, Studer M. Area-Specific temporal control of corticospinal motor neuron differentiation by COUP-TF1. Feb 2. [Epub ahead of print], 2010, PNAS.
- Cotugno G, Tessitore A, Annunziata P, Strisciuglio C, Faella A, Aurillo M, Di Tommaso M, Russo F, Mancini A, De Leonibus E, Aloj L, Auricchio A. Different serum enzyme levels are required for the rescue of the various systemic features in mucopolysaccharidoses. Dec 18, 2009, Hum Gene Ther.
- Manago’ F, Castellano C, Oliverio A, Mele A, De Leonibus E. Role of dopamine receptors subtypes, D1-like and D2-like, within the nucleus accumbens subregions, core and shell, on memory consolidation in the one-trial inhibitory avoidance task. 16(1):46-52, 2009, Learning & Memory.
- De Leonibus E, Managò F, Giordani F, Petrosino F, Lopez S, Oliverio A, Amalric M, Mele A. Metabotropic glutamate receptors 5 blockade reverses spatial memory deficits in a mouse model of Parkinson disease. 34(3):729-38, 2009, Neuropsychopharmacology.
- Verheij MM, de Mulder EL, De Leonibus E, van Loo KM, Cools AR. Rats that differentially respond to cocaine differ in their dopaminergic storage capacity of the nucleus accumbens. Mar 1, 2008, J Neurochem.
- Lopez S, Turle-Lorenzo N, De Leonibus E, Mele A, Amalric M. Targeting group III metabotropic glutamate receptors in Parkinsonian rats produces opposite behavioral effects in the direct and indirect pathways of the basal ganglia. Jun 20;27(25):6701-11, 2007, Journal Neuroscience.
- De Leonibus E, Pascucci T, Lopez S, Amalric M, Oliverio A, Mele A. Visuo-spatial deficits in animal models of Parkinson disease Jul 11, 2007, Psychopharmachology.
- De Leonibus E, Verheij MMM, Mele A, Cools AR. Distinct kinds of novelty processing differentially increase extracellular dopamine in different brain regions. 2006; 23(5):1332-40, Eur J Neurosci.
- De Leonibus E, Oliverio A, Mele A. A study on the role of the dorsal striatum and the nucleus accumbens in allocentric and egocentric spatial memory consolidation. 2005; 12(5): 491-503. Epub 2005 Sep 15. Learning & Memory JOURNAL COVER.
- Ferretti V, Florian C, Costantini VJ, Roullet P, Rinaldi A, De Leonibus E, Oliverio A, Mele A. Co-activation of glutamate and dopamine receptors within the nucleus accumbens is required for spatial memory consolidation in mice. 2005, 179: 108-116. Psychopharmacology (Berl).
41. Mele A, Avena M, Roullet P, De Leonibus E, Mandillo S, Sargolini F, Coccurello R, Oliverio A. Nucleus accumbens dopamine receptors in the consolidation of spatial memory. 2004 Sep;15(5-6):423-31 Behav Pharmacol.
- De Leonibus E, Costantini VJ, Castellano C, Ferretti V, Oliverio A, Mele A. Distinct roles of the different ionotropic glutamate receptors within the nucleus accumbens in passive-avoidance learning and memory in mice. 2003 Oct;18(8):2365-73. Eur J Neurosci.
- De Leonibus E, Lafenetre P, Oliverio A, Mele A. Pharmacological evidence of the role of dorsal striatum in spatial memory consolidation in mice. 2003 Aug;117(4):685-94 Behav Neurosci.
- De Leonibus E, Mele A, Oliverio A, Pert A. Distinct pattern of c-fos mRNA expression after systemic and intra-accumbens amphetamine and MK-801. 2002;115(1):67-78. Neuroscience.
- De Leonibus E, Mele A, Oliverio A, Pert A. Locomotor activity induced by the non-competitive N-methyl-D-aspartate antagonist, MK-801: role of nucleus accumbens efferent pathways. 2001;104(1):105-16. Neuroscience
*Identical contribution to the study

Post- Docs:
Giulia Torromino, Phd
Giulia.torromino@cnr.it
Giulia is interested in the neurobiology of learning and memory, focusing on the interaction between cortical and subcortical brain regions in memory formation, in normal and pathological conditions including ageing and related neurodegenerative disorders. Giulia is currently focusing on sex-differences in brain wiring and in neuronal computation of different types of memories. To address this complex issue, Giulia combines in vivo electrophysiology, behavioural pharmacologenetics and optogenetics, with ex-vivo imaging.
Maria De Risi, PhD
Maria.derisi@cnr.it
Maria is interested in the behavioural and molecular pharmacology of neuropsychatric and neurodegenerative disorders with a particular focus on dopamine/glutamate signalling in Parkinson’ disease, ageing and lysosomal storage disorders. Through a combination of in vivo, ex vivo and cellular approaches she is working to identify early disease pathways in to target with small molecules. Maria works in a joint lab with TIGEM (https://www.tigem.it/research/faculty/deleonibus)
Vittorio Loffredo, PhD
loffredo.1756114@studenti.uniroma1.it
Vittorio is working on sex differences in ageing and Parkinson’ disease the sex-specific effects of behavioural and pharmacological treatments for cognitive and synaptic plasticity impairments.
Post-graduate fellow:
Ylenia Gigante
y.gigante@tigem.it
Master thesis students:
Diletta Cavezza
cavezza.1645636@studenti.uniroma1.it
Greta Fabiani
fabiani.1545968@studenti.uniroma1.it
Brunella Mongiardi
brunellamongiardi95@gmail.com
Francesco Crupi
francesco.crupi96@gmail.com
Chiara Di Marino
c.dimarino@tigem.it
2019-2022 Italian Ministery of University (PRIN, MUR): Role of alpha-synuclein and LRRK2 in Levodopa-Induced Dyskinesia; Role: Unit PI
2016-2020 Italian Ministery of University (PRIN, MUR): Targeting early synaptic dysfunctions induced by alpha-synuclein as a novel therapeutic approach in Parkinson’s disease; Role: Unit PI
2016-2020 Alzheimer’s Association(https://www.alz.org/): Neurobiology of sex differences in memory capacity in ageing and AD; Role: PI
2019-2020 Sanfilippo Children’s Foundation (https://www.sanfilippo.org.au/): New therapeutic strategies for the treatment of behavioural symptoms in MPS-IIIA; Role: PI
2017-2019 National MPS Society (https://mpssociety.org/): Disease’ mechanisms leading to dopaminergic dysfunction underlying behavioural symptoms in MPSIIIA; Role: PI
2015-2018 Italian Ministery of University (The Aging Project, MUR): Disease mechanisms of working memory load capacity in ageing and Alzheimer’ disease; Role: Coordinator/PI
2013-2016 Fondazione Con il Sud (https://www.fondazioneconilsud.it/): New markers for dementia: a multidisciplinary approach; Role: Coordinator/PI
2010-2013 Alzheimer’s Association(https://www.alz.org/): Neurobiology of working memory span in normal and pathological ageing; Role: PI
- 17/03/2021 Intervista radio: https://echannel.enel.com/dem/podcast/abc-digital-memoria-e-tech-intervista-cnr-de-leonibus
- 05/01/2021 Intervista TV: SIAMO NOI (Ora: 15:51:59 Min: 3:32)
- https://www.pfizerpro.it/news/medicina-molecola-anti-et-ripulisce-ingranaggi-ingolfati-della-memoria
- 07/08/2020 it SPERMIDINA, LA MOLECOLA CHE MIGLIORA LA MEMORIA
- 07/08/2020 it MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 07/08/2020 com 07/08/2020 LA MOLECOLA CHE RIPULISCE GLI INGRANAGGI DELLA MEMORIA
- 07/08/2020 com SCOPERTA CNR: MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGIINGOLFATI DELLA MEMORIA
- 07/08/2020 it MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 07/08/2020 corrierenazionale.it STUDIO HA DIMOSTRATO CHE LA SPERMIDINA, UNA SOSTANZAPRESENTE NATURALMENTE IN MOLTI CIBI…
- 07/08/2020 com SPERMIDINA, LA MOLECOLA CHE FAVORISCE LA LONGEVITA’ ERIPULISCE GLI INGRANAGGI DELLA MEMORIA
- 07/08/2020 com SPERMIDINA, LA MOLECOLA CHE FAVORISCE LA LONGEVITA’ ERIPULISCE GLI INGRANAGGI DELLA MEMORIA
- 07/08/2020 Sassari Notizie.com | SALUTE | MOLECOLA ANTI-ETA’ RIPULISCEINGRANAGGI INGOLFATI DELLA MEMORIA
- 06/08/2020 com SCOPERTA CNR: MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGIINGOLFATI DELLA MEMORIA
- 06/08/2020 it MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 06/08/2020 it ECCO LA MOLECOLA CHE RIPULISCE GLI INGRANAGGI DELLAMEMORIA
- 06/08/2020 Ansa.it ECCO LA MOLECOLA CHE RIPULISCE GLI INGRANAGGI DELLAMEMORIA
- 06/08/2020 It MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 06/08/2020 it RICERCA. LA MOLECOLA CHE RIPULISCE GLI INGRANAGGI DELLAMEMORIA35Gds.it
- 06/08/2020ECCO LA MOLECOLA CHE RIPULISCE GLI INGRANAGGI DELLAMEMORIA
- 06/08/2020 it ECCO LA MOLECOLA CHE RIPULISCE GLI INGRANAGGI DELLAMEMORIA
- 06/08/2020 news MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 06/08/2020 it MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 06/08/2020 it SPERMIDINA, LA MOLECOLA IN GRADO DI CORREGGERE I DIFETTIDELLA MEMORIA
- 06/08/2020 it DECLINO COGNITIVO, MOLECOLA PRESENTE IN MOLTI CIBIRIPULISCE GLI INGRANAGGI DELLA MEMORIA
- 06/08/2020 finance.yahoo.com SCOPERTA CNR: MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGIINGOLFATI DELLA MEMORIA
- 06/08/2020 Yahoo.Com SCOPERTA CNR: MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGIINGOLFATI DELLA MEMORIA
- 06/08/2020 it MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 06/08/2020 it MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 06/08/2020 it MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 06/08/2020 it LA MOLECOLA CHE RIPULISCE GLI INGRANAGGI DELLA MEMORIA
- 06/08/2020 eu LA MOLECOLA CHE RIPULISCE GLI INGRANAGGI DELLA MEMORIA
- 06/08/2020 it MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 06/08/2020 info LA MEMORIA FA CILECCA? SCOPERTA UN MOLECOLA CHERIPULISCE GLI INGRANAGGI INCEPPATI
- 06/08/2020 it SCOPERTA CNR: MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGIINGOLFATI DELLA MEMORIA
- 06/08/2020 it SCOPERTA LA MOLECOLA CHE RIPULISCE GLI INGRANAGGI DELLAMEMORIA
- 06/08/2020 com MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 06/08/2020 It LA MOLECOLA CHE RIPULISCE GLI INGRANAGGI DELLA MEMORIA
- 06/08/2020 it MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 06/08/2020 it LA MOLECOLA CHE RIPULISCE LA NOSTRA MEMORIA
- 06/08/2020 it MOLECOLA ANTI-ETA’ RIPULISCE INGRANAGGI INGOLFATI DELLAMEMORIA
- 06/08/2020 it LA MOLECOLA CHE RIPULISCE GLI INGRANAGGI DELLA MEMORIA
- Science Cafè Seminar “La diversità nel cervello delle donne: un universo ancora da scoprire”, Caffè Letterario, Esperimenti per una nuova cultura, Busto Arsizio, Italy, 12 March, 2020.
- Lecture for secondary school “Il cervello e le sue dipendenze”. Istituto Comprensivo A. Balabanoff, 19 Febuary 2020, Rome, Italy.
- Science at Kinder garden: “esperimenti di colori e odori al nido”, Kinder Garden, 4 November 2019, Rome, Italy.
- Television Interview: “Identificazione nuovi marker per la malattia di Parkinson”, December 2017, Telethon Marathon National RAI1 Program.
- Seminar “Il GPS del cervello” MEMOFEST 2016, Massa, Italy 3rd June 2016
- Seminar “The brain and its memories”. Futuro in Ricerca year event, Naples, 14 October 2015
- Seminar “The brain and its memories”. Futuro in Ricerca year event, Naples, 14 October 2015
- Lecture for primary school “The scientific method”. Elementary School “Nostra Signora della Neve”, Rome, Italy.
- Seminar: “Invecchiamento e desclino cognitivo: i campanelli d’allarme” “IL SORRISO DI CANDIDA short-MOVIE”. Quando la vita incrocia l’Alzheimer., Castello “Dentice di Frasso”, CAROVIGNO (BR), Italy, 18th September, 2014.
- Interview, “Dementia”, regional radio program “Genetics Today” on Radio Cusano Campus (100 Fm, Rome and Lazio: http://www.radiocusanocampus.it/podcast/?prog=130). 19th September 2014.
- Seminar: “Invecchiamento e declino cognitivo: i campanelli d’allarme” Festa della Donna “Women’s party”. Città della Scienza, Naples, Italy, 8th March 2014.
- Article-Inverview: “Demenza, quando il malato si ‘nasconde’ (http://www.almanacco.cnr.it/reader/cw_usr_view_articolo.html?id_articolo=5472&giornale=5456). Almanacco della Scienza, March 2014.
- Seminar “Quanti oggetti possiamo stipare nel magazzino delle nostre memorie”, Liberamente “Tastes of Neuroscience”, Rome, Italy, 20th April, 2012.
- Interview: “Il cervello è il mio mestiere”, di Daniela Mattialia, Panorama (National magazine), 11st December, 2008.
- Television Interview: “Brain Drain of a young neuroscience scientist”, December 2010, National RAI1 program.
- Lecture for secondary school: “how emotions regulate our behavior”. Secondary public schools in Naples, 2010.
- “Brain at school”. Brain Awareness Week (BAW), 18-20 March, Naples, Italy, 2009. Co-organizer.
- Seminar: “Il cervello e le sue memorie”. Brain Awarness Week (BAW), 18-20 March, Naples, Italy, 2009.
- Seminar: “Neurobiology of Novelty”. Rotary club Nijmegen, The Netherlands, 22nd April 2004.
